Technology & Innovation – Abbott

Abbott’s innovative technology is helping people of all ages living with diabetes live life to the fullest. Hear how it’s helping Michaela…
Better diabetes management1-3
Management of diabetes has become more challenging over the last few years. To help people living with diabetes get access to the right technology, resources and information, the National Institute for Health and Care Excellence (NICE) has updated its guidance.
The latest guidelines address current inequalities in flash and continuous glucose monitoring (CGM) by recommending wider access on the NHS for adults and children with type 1 diabetes and people with type 2 diabetes on insulin-intensive therapy who meet defined criteria.
• Adults with type 1 diabetes.4
• Children and young people with type 1 and type 2 diabetes.5
• People with type 2 diabetes on insulin-intensive therapy who meet defined criteria.6
You may wish to discuss these new guidelines with your diabetes team at your next appointment. However, it is important to remember that local areas are still working through their implementation.
Read more about the UK guidance here. Ireland has different guidance, which can be found here.
Abbott’s FreeStyle Libre 2 flash glucose monitoring system
The FreeStyle Libre 2 system is the #1 sensor-based glucose monitoring system used worldwide7. Here’s why:
- The small, discreet sensor is easily attached to the back of the upper arm, where it continuously measures glucose levels.
- It’s worn for up to 14 days and is water-resistant8, so it continues to work during exercise, swimming or showering.
- Accuracy overall is excellent, even in the low glucose range when it matters the most.9

- A painless3 1-second scan enables you to check glucose levels anytime, anywhere, even through clothing.
- The display shows current glucose reading, 8-hour history and a trend arrow so you can take appropriate action.
- Optional glucose alarms can be set according to your needs to alert you when your glucose is too high or too low.
Discover more about the FreeStyle Libre 2 system here

Get the most out of the FreeStyle Libre 2 system by connecting with the digital health solutions
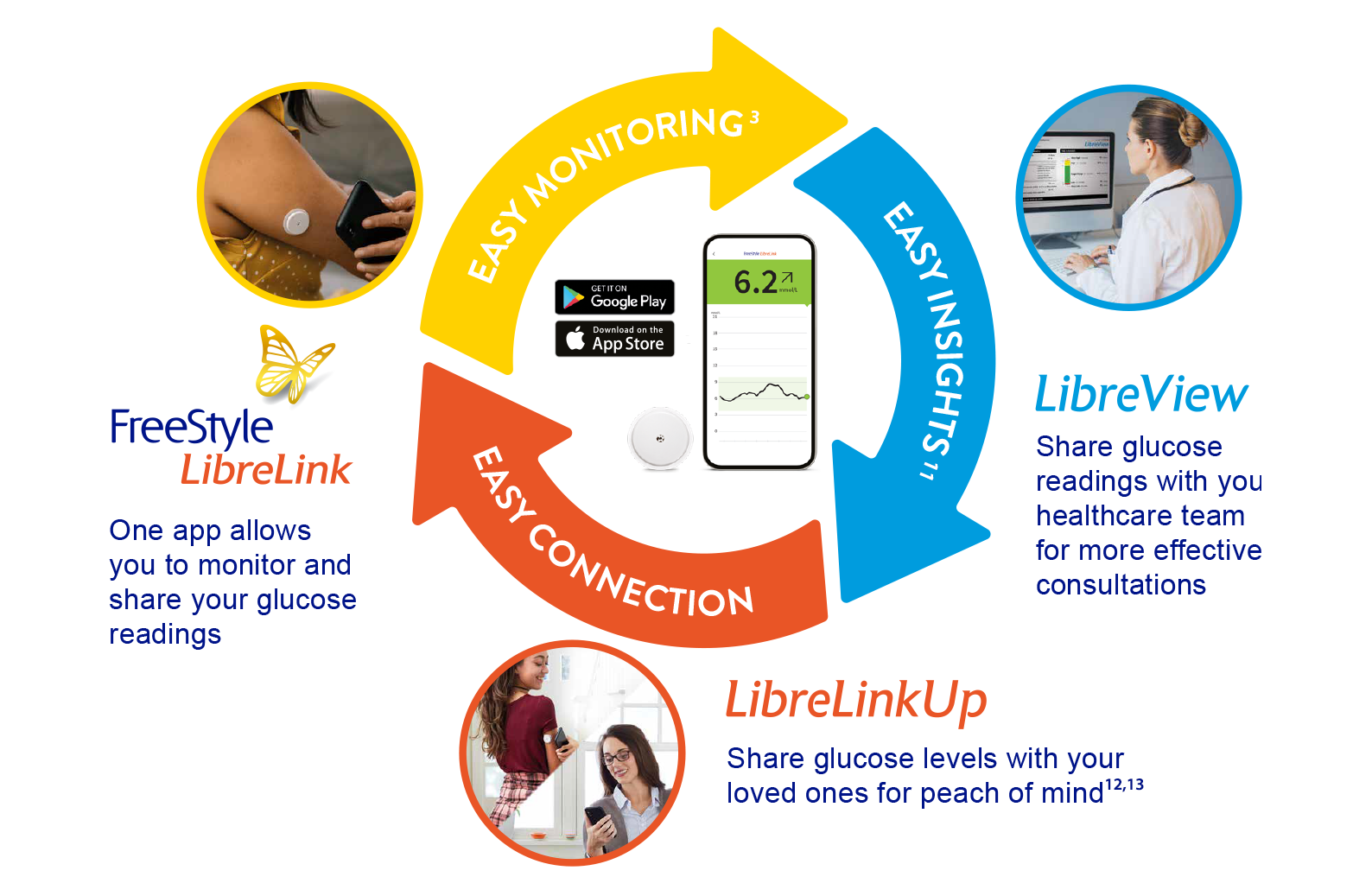
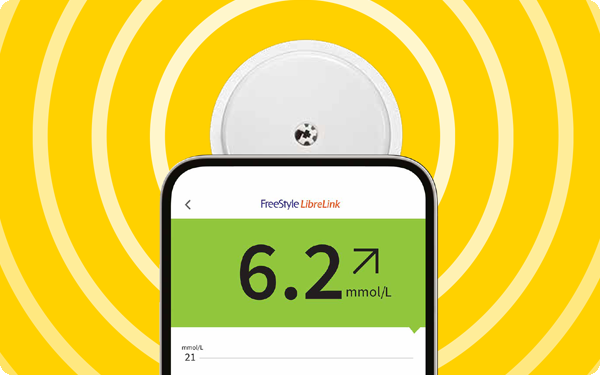
Easy monitoring with the FreeStyle LibreLink app14
Get your glucose readings anytime10, anywhere8
The FreeStyle LibreLink app enables you to use your phone to scan your FreeStyle Libre 2 sensor.
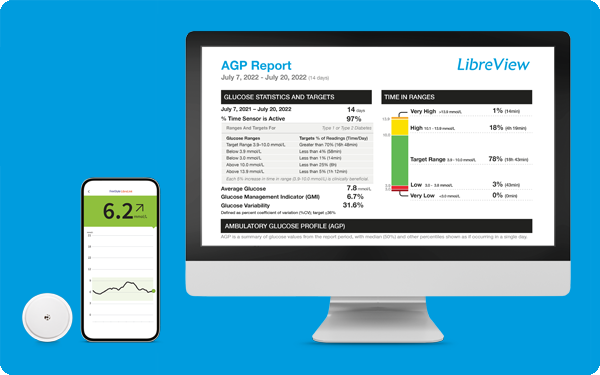
Easy insights with LibreView11
Enable better informed diabetes management decisions
LibreView is a free, cloud-based diabetes management system that allows you to share your reports with your healthcare professional securely and confidently.15
Find out more about LibreView here.
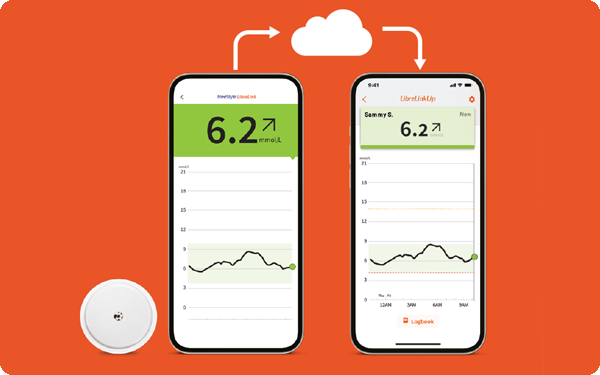
Easy connection with LibreLinkUp12
Let family and friends help you manage your diabetes
With the LibreLinkUp mobile app, you can remotely share your glucose levels with your caregivers and loved ones for added peace of mind.
Get connected with the LibreLinkUp app here.
The FreeStyle LibreLink app and LibreLinkUp app are available for Android and iPhone
Check if you have a compatible phone with this smartphone compatibility guide.

Visit out website www.FreeStyleLibre.co.uk for more information and resources
Images are for illustrative purposes only. Not actual patient or data.
1. Bolinder, J. Lancet (2016): http://doi.org/10.1016/S0140-6736(16)31535-5.
2. Evans, M. Diabetes Ther (2020): https://doi.org/10.1007/s13300-019-00720-0.
3. Haak, T., Diabetes Ther. (2017) : https://doi.org/10.1007/s13300-016-0223-6.
4. NICE guideline NG17 (2015) Last updated: 17th Aug 2022 , available at https://www.nice.org.uk/guidance/ng17.
5. NICE guideline NG18 (2015) Last updated 29th June 2022 available at https://www.nice.org.uk/guidance/ng18.
6. NICE NG28 (2015) Last updated 29th June 2022 available at: https://www.nice.org.uk/guidance/ng28/resources/type-2-diabetes-in-adults-managementpdf-1837338615493.
7. Data on file, Abbott Diabetes Care. Data based on the number of users worldwide for the FreeStyle Libre system compared to the number of users for other leading personal use sensor based glucose monitoring systems.
8. Sensor is water-resistant in up to 1 meter (3 feet) of water. Do not immerse longer than 30 minutes. Not to be used above 10,000 feet.
9. Alva, S. et al. Journal of Diabetes Science and Technology, (September 2020). http://doi.org/10.1177/1932296820958754.
10. 60-minute warm-up required when applying the sensor.
11. Unger, J., Postgrad Med. (2020): https://doi.org/10.1080/00325481.2020.1744393.
12. The LibreLinkUp app is only compatible with certain mobile device and operating systems. Please check www.LibreLinkUp.com for more information about device compatibility before using the app. Use of LibreLinkUp and FreeStyle LibreLink requires registration with LibreView. The LibreLinkUp mobile app is not intended to be a primary glucose monitor: home users must consult their primary device(s) and consult a healthcare professional before making any medical interpretation and therapy adjustments from the information provided by the app.
13. Campbell, F. Pediatr. Diabetes (2018): https://doi.org/10.1111/pedi.12735.
14. The FreeStyle LibreLink app is only compatible with certain mobile devices and operating systems. Please check the website for more information about device compatibility before using the app. Use of FreeStyle LibreLink requires registration with LibreView.
15. The LibreView website is only compatible with certain operating systems and browsers. Please check www.LibreView.com for additional information.
© 2023 Abbott. FreeStyle, Libre, and related brand marks are marks of Abbott. ADC-34094 v2 02/23.
